
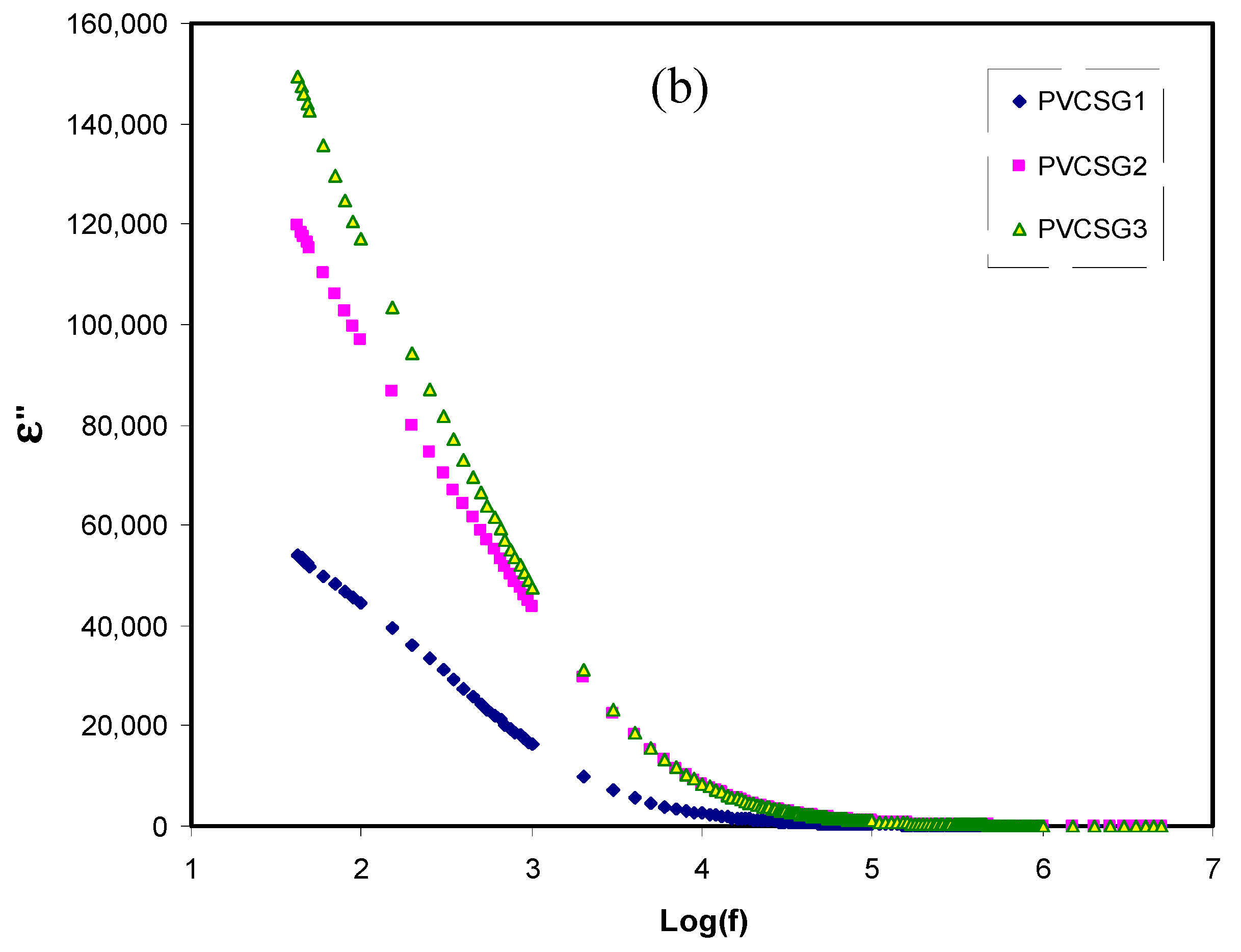
Carrageenans form strong interpolymer complexes through hydrogen bonding with poly (vinyl alcohol), these complexes imparting higher tensile strength and improved water barrier properties to PVA films in comparison with other biopolymers such as Na-alginate, gelatin, chitosan or carboxymethylcellulose. This class of biopolymers possesses a high tendency to form thermoreversible gels through extended hydrogen bonding between molecules adopting a single-helix conformation (λ) or between double-helix molecular associations (κ and ι carrageenans). Supplementary, this addition modulates the release of ionic or polar active principles (antibiotics, nutraceuticals, and other drugs) in controlled-release applications, while also extending or improving the performance of the PVA hydrogel matrix in conjunction with the sorption of potentially harmful species (e.g., dyes, heavy metal cations, anions, and pesticides ) for environmental remediation applications.Ĭarrageenans represent a class of linear water-soluble sulfated galactan polysaccharides that are isolated mainly from marine red algae. Moreover, the addition of polysaccharides with ionized or ionizable groups to PVA, for example chitosan, alginates, carrageenan, pectin, pectinates, or modified cellulose, leads to the formation of hydrogels with new functional properties (antimicrobial materials, sensors ). It also improves the flexibility, hardness, and compression resistance of the PVA-blend hydrogels through the creation of extended crosslinking points in the polymer matrix.

īlending PVA with biopolymers (especially polysaccharides) increases the biocompatibility, hydrophilicity, and swelling of hydrogels, through the increase in the density of the hydrophilic groups. The second method to tune the properties of PVA hydrogels (applied either standalone or in combination with the first) is represented by PVA compounding with various inorganic or organic compounds, respectively, blending with synthetic and/or natural polymers. PVA chemical modification and crosslinking represent one of the two primary tools through which the solubility, porosity, diffusion, swelling, sorption yield, and hydrophilicity of PVA hydrogels could be tuned to match various applicative demands from both research and industry. Poly (vinyl alcohol) (PVA) has been widely in used in the formulation of hydrogel matrices for controlled-release or environmental remediation applications since the early 1960s, due to its nontoxicity, biocompatibility, and to the availability of an extensive palette of chemical modification (functionalization) and crosslinking reactions.


 0 kommentar(er)
0 kommentar(er)
